
Chemistry, 08.04.2020 22:29 ayoismeisalex
Given the balanced equation:
ZnSO4 + SrCl2 > SrSO4 + ZnCl2
What number of moles of SrCl2 is consumed when 54 g of ZnCl2 is produced?
a) 0.16 b) 0.3 c) 0.79 d) 1.58 e) 0.4

Answers: 1


Another question on Chemistry

Chemistry, 20.06.2019 18:04
Why is it illegal to manufacture fireworks without a license
Answers: 1

Chemistry, 22.06.2019 05:20
Identify and describe the three ways that mutations affect organisms.
Answers: 1

Chemistry, 22.06.2019 09:30
What are scientists who study fossils called? ( a ) astronomers. ( b ) biologists. ( c ) geologists. ( d ) paleontologists.
Answers: 2

Chemistry, 22.06.2019 09:40
Apiece of copper has a temperature of 75.6 0c. when the metal is placed in 100.0 grams of water at 19.1 0c, the temperature rises by 5.5 0c. what is the mass of the metal?
Answers: 1
You know the right answer?
Given the balanced equation:
ZnSO4 + SrCl2 > SrSO4 + ZnCl2
What number...
ZnSO4 + SrCl2 > SrSO4 + ZnCl2
What number...
Questions



Social Studies, 14.11.2019 05:31



History, 14.11.2019 05:31

English, 14.11.2019 05:31

Mathematics, 14.11.2019 05:31

Mathematics, 14.11.2019 05:31




Mathematics, 14.11.2019 05:31




Mathematics, 14.11.2019 05:31


English, 14.11.2019 05:31

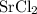 . consumed by 54 grams of zinc chloride has been 0.4 moles. Thus the correct option is e.
. consumed by 54 grams of zinc chloride has been 0.4 moles. Thus the correct option is e.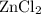 consumes 1 mole of
consumes 1 mole of 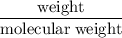
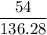 mol
mol

