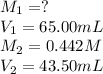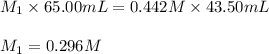Chemistry, 09.04.2020 00:26 mairadua14
A standard solution of 0.442 M NaOH was used to determine the concentration of a hydrochloric acid solution. If 43.50 mL of NaOH is needed to neutralize 65.00 mL of the acid, what is the molar concentration of the acid in the original solution

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 23:00
What is the molecular formula for a compound that is 46.16% carbon, 5.16% hydrogen, and 48.68% fluorine? the molar mass of the compound is 156.12 g/mol
Answers: 2

Chemistry, 22.06.2019 05:20
Temperature is _related to the average kinetic energy of a gas. inversely directly not disproportionally
Answers: 1

Chemistry, 22.06.2019 06:40
Which statement is usually true about the relationship between activation energy and reaction rates? low activation energy barriers result in low rates. high activation energy barriers result in low rates. low activation energy barriers result in no reaction. high activation energy barriers result in no reaction.
Answers: 3

You know the right answer?
A standard solution of 0.442 M NaOH was used to determine the concentration of a hydrochloric acid s...
Questions






Physics, 05.07.2019 00:30









Chemistry, 05.07.2019 00:30





Physics, 05.07.2019 00:30

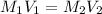
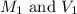 are the initial molarity and volume of HCl.
are the initial molarity and volume of HCl.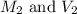 are the final molarity and volume of NaOH.
are the final molarity and volume of NaOH.