of aluminum are placed in a hot water


Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 15:20
Which description best characterizes the motion of particles in a solid?
Answers: 2

Chemistry, 22.06.2019 16:00
What rule is used to determine how many covalent bonds an element can form? a. the number of covalent bonds is equal to six c the number of covalent bonds is equal to five minus the group number plus the group number b. the number of covalent bonds is equal to eight d. none of the above minus the group number select the best answer from the choices provided
Answers: 2

Chemistry, 22.06.2019 20:00
There are two steps in the usual industrial preparation of acrylic acid, the immediate precursor of several useful plastics. in the first step, calcium carbide and water react to form acetylene and calcium hydroxide: cac2 (s) + 2h2o (g) → c2h2 (g) + caoh2 (s) =δh−414.kj in the second step, acetylene, carbon dioxide and water react to form acrylic acid: 6c2h2 (g) + 3co2 (g) + 4h2o (g) → 5ch2chco2h (g) =δh132.kj calculate the net change in enthalpy for the formation of one mole of acrylic acid from calcium carbide, water and carbon dioxide from these reactions. round your answer to the nearest kj .
Answers: 3

Chemistry, 22.06.2019 20:00
How are the terms group and period used on the periodic table
Answers: 1
You know the right answer?
A 175 g piece of iron and a 175 g piece
of aluminum are placed in a hot water
of aluminum are placed in a hot water
Questions

Mathematics, 24.05.2021 01:00

Mathematics, 24.05.2021 01:00

Mathematics, 24.05.2021 01:00


Mathematics, 24.05.2021 01:00





Mathematics, 24.05.2021 01:00



Geography, 24.05.2021 01:00




Mathematics, 24.05.2021 01:00


Mathematics, 24.05.2021 01:00

Mathematics, 24.05.2021 01:00

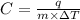
 = change in temperature of substance
= change in temperature of substance

