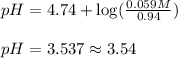Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 02:30
In pea plants, the allele for tallness (t) is dominant to the allele for shortness (t). in the cross between a tall pea plant and a short pea plant shown below, what is the probability that the resulting offspring will be tall? whats the percent
Answers: 1

Chemistry, 22.06.2019 06:30
If 1.8 l of water is added to 2.5l of a 7.0 molarity koh solution, what is the molarity of the new solution
Answers: 1

Chemistry, 22.06.2019 17:00
In a heat engine of 1000 j of heat enters the system and the piston does 500 j of work what is the final internal energy of the system if the inital energy was 2000 j we have to do all of these down here 1)write the equation 2)list out your know variables 3)plug the numbers into the equations 4)solve 5)write your solution statemtn that includes inital energuy and final energuy added
Answers: 1

Chemistry, 22.06.2019 17:30
In a heat of an engine, if 700 j enters the system, and the piston does 400 j of work what is the final internal (thermal) energy of the system if the initial energy is 1,500 j
Answers: 2
You know the right answer?
Calculate the ph of a buffer that is 0.94 mhc2h3o2 and 0.059 mnac2h3o2. the ka for hc2h3o2 is 1.8×10...
Questions


Mathematics, 14.04.2020 15:53

History, 14.04.2020 15:53






English, 14.04.2020 15:53



Mathematics, 14.04.2020 15:53

Mathematics, 14.04.2020 15:53






Mathematics, 14.04.2020 15:53

![pH=pK_a+\log(\frac{[salt]}{[acid]})](/tpl/images/0591/1390/e4eea.png)
![pH=pK_a+\log(\frac{[NaHCO_3]}{[H_2CO_3]})](/tpl/images/0591/1390/c2e30.png)
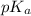 = negative logarithm of acid dissociation constant of carbonic acid
= negative logarithm of acid dissociation constant of carbonic acid ![pK_a=-\log[K_a]=-\[1.8\times 10^{-5}]=4.74](/tpl/images/0591/1390/24626.png)
![[NaC_2H_3O_2]=0.059 M](/tpl/images/0591/1390/dff64.png)
![[HC_2H_3O_2]=0.94 M](/tpl/images/0591/1390/33ac1.png)