Chemistry, 09.04.2020 03:05 trevorhenyan51
Calculate [H3O+] at 25 ∘C for each solution and determine if the solution is acidic, basic, or neutral. a.) [OH−] = 3.8×10−2 Mb.) [OH−] = 1.0×10-7 Mc.) [OH−] = 5.5×10−10 MPlease show the work and how you determine if the solution is neutral, acidic, or basic

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 05:00
In 1901, thomas edison invented the nickel-iron battery. the following reaction takes place in the battery. fe(s) + 2 nio(oh)(s) + 2 h2o(l) fe(oh)2(s) + 2 ni(oh)2(aq) how many mole of fe(oh)2, is produced when 5.35 mol fe and 7.65 mol nio(oh) react?
Answers: 1

Chemistry, 22.06.2019 14:10
Aconcentrated solution of ammonia is 14.8m and has a density of 0.899g/l. what is the concentration of ammonia in this solution in weight percent (%w/w)?
Answers: 1

Chemistry, 22.06.2019 19:40
What is the wavelength of a 3*10^12 hz infrared wave a 3*10^20m b 1* 10^4m c 3*10^-3m d 1*10^-4 m
Answers: 1

Chemistry, 23.06.2019 07:00
In order for a high temperature boiler or steam engine to produce superheated water, or steam: the heat source must be greater than 100°c the water must be permitted to evaporate quickly the system must be sealed and become pressurized above atmospheric pressure the vapor pressure must be kept below 760 mm(hg)
Answers: 1
You know the right answer?
Calculate [H3O+] at 25 ∘C for each solution and determine if the solution is acidic, basic, or neutr...
Questions

History, 24.07.2019 04:20

Chemistry, 24.07.2019 04:20

SAT, 24.07.2019 04:20

Geography, 24.07.2019 04:20




History, 24.07.2019 04:20

Mathematics, 24.07.2019 04:20

Mathematics, 24.07.2019 04:20

Mathematics, 24.07.2019 04:20


Physics, 24.07.2019 04:20

Mathematics, 24.07.2019 04:20




Mathematics, 24.07.2019 04:20


Mathematics, 24.07.2019 04:20

![pH=-log[H_3O^+]](/tpl/images/0591/2031/6e71a.png)
![[H_3O^+][OH^-]=K_w=10^{-14}](/tpl/images/0591/2031/b8614.png) [ at 25°C]
[ at 25°C]![log[H_3O^+]+log[OH^-]=logK_w=log 10^{-14}](/tpl/images/0591/2031/bb8c2.png)
![\Rightarrow - log[H_3O^+]-log[OH^-]=-logK_w=-log 10^{-14}](/tpl/images/0591/2031/a8528.png)
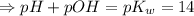
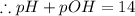
![[OH^-]=3.8\times10^{-2}M](/tpl/images/0591/2031/42d58.png)
![- log[H_3O^+]-log[OH^-]=14}](/tpl/images/0591/2031/0c29a.png)
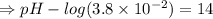
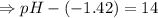
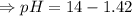
![[OH^-]=1.0\times10^{-7}M](/tpl/images/0591/2031/ac083.png)
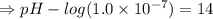
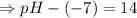
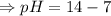
![[OH^-]=5.5\times10^{-10}M](/tpl/images/0591/2031/f6aae.png)