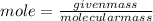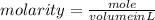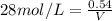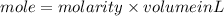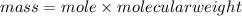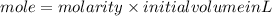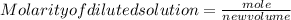1. What volume in mL of 28.0M H2SO4 is needed to contain 53.0g of H2SO4?
2. How many gra...

1. What volume in mL of 28.0M H2SO4 is needed to contain 53.0g of H2SO4?
2. How many grams of calcium hydroxide Ca(OH)2 are needed to make 532.0 mL of a 1.90M solution?
3. If 12 mL of water are added to 124mL of a 0.505M K2SO4 solution, what will the molarity of the diluted solution be?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 08:00
Which of the following observations indicates that there is a small, dense, positively charged part in the center of an atom? some uncharged particles are scattered by a gold foil. all uncharged particles are attracted towards a gold foil. all positively charged particles pass straight through a gold foil. some positively charged particles bounce back from a gold foil.
Answers: 2

Chemistry, 22.06.2019 12:00
Give the set of reactants (including an alkyl halide and a nucleophile) that could be used to synthesize the following ether: draw the molecules on the canvas by choosing buttons from the tools (for bonds and charges), atoms, and templates toolbars, including charges where needed. ch3ch2och2ch2chch3 | ch3
Answers: 1

Chemistry, 22.06.2019 13:10
What type of interaction occurs between the r groups of valine and isoleucine in a tertiary structure? view available hint(s) what type of interaction occurs between the r groups of valine and isoleucine in a tertiary structure? salt bridge disulfide bridge hydrogen bond hydrophobic interaction
Answers: 1

You know the right answer?
Questions


Social Studies, 19.02.2021 18:50

Mathematics, 19.02.2021 18:50

Business, 19.02.2021 18:50





Spanish, 19.02.2021 18:50

Mathematics, 19.02.2021 18:50

English, 19.02.2021 18:50

History, 19.02.2021 18:50


Biology, 19.02.2021 18:50



English, 19.02.2021 18:50

Mathematics, 19.02.2021 18:50


Mathematics, 19.02.2021 18:50