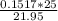Chemistry, 09.04.2020 04:37 Bearboy5957
A buret is filled with 0.1517 m naoh (aq). 25.0 ml of an unknown acid ha (aq) and two drops of indicator are added to an erlenmeyer flask, and the titration experiment is carried out. if the initial buret reading was 0.55 ml, and the buret reading at the endpoint was 22.50 ml, what is the molarity of the unknown acid, ha?

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 03:50
Which of the following statements about acidic water is true? a. acid has no effect on the h,o molecules. b. the solution contains a larger number of oh ions than h,o ions. c. the solution contains a larger number of h,o ions than qh ions. d. the solution contains an equal number of h,o ions and oh ions. none of the above e.
Answers: 1


Chemistry, 22.06.2019 20:00
What happens to the temperature of a substance when the average kinetic energy of its particles increases?
Answers: 3
You know the right answer?
A buret is filled with 0.1517 m naoh (aq). 25.0 ml of an unknown acid ha (aq) and two drops of indic...
Questions


History, 14.12.2020 23:10



Social Studies, 14.12.2020 23:10

English, 14.12.2020 23:10



Mathematics, 14.12.2020 23:10

Biology, 14.12.2020 23:10

Mathematics, 14.12.2020 23:10


English, 14.12.2020 23:10


Mathematics, 14.12.2020 23:10

Mathematics, 14.12.2020 23:10

Health, 14.12.2020 23:10

English, 14.12.2020 23:10

Mathematics, 14.12.2020 23:10

Mathematics, 14.12.2020 23:10

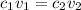 where 1 and 2 are the values for acid and base respectively.
where 1 and 2 are the values for acid and base respectively.