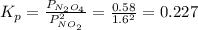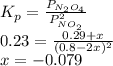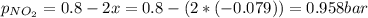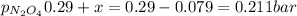N2O4 decomposes into NO2. At a certain temperature, the equilibrium pressures of NO2 and N2O4 are 1.6 bar and 0.58 bar, respectively. If the volume of the container is doubled at constant temperature, what would be the partial pressures of the gases when equilibrium is re-established

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 11:00
Which statement is true about hcl? (5 points) select one: a. it is a salt because it increases the concentration of metallic ions. b. it is a salt because it is formed by the reaction of an acid and a base. c. it is an acid because it increases the concentration of hydroxyl ions. d. it is an acid because it increases the concentration of hydronium ions.
Answers: 1

Chemistry, 22.06.2019 13:00
Imagine that you push on a large rock. at what point does your effort change the rock’s mechanical energy?
Answers: 1

Chemistry, 22.06.2019 22:00
11) burning your hand when accidentally touching a hot plate is an example of which heat transfer? a. conduction b. convection c. radiation d. none of these
Answers: 2

Chemistry, 22.06.2019 23:30
The comparison of the number of atoms in a copper coin the size of a penny with the number of people on earth is made to illustrate which of the following? a. that atoms are indivisible b. that atoms are very small c. that atoms are very large d. that in a copper penny, there is one atom for every person on earth
Answers: 1
You know the right answer?
N2O4 decomposes into NO2. At a certain temperature, the equilibrium pressures of NO2 and N2O4 are 1....
Questions







Mathematics, 26.06.2020 15:01



Mathematics, 26.06.2020 15:01


Mathematics, 26.06.2020 15:01





Mathematics, 26.06.2020 16:01

Mathematics, 26.06.2020 16:01

Mathematics, 26.06.2020 16:01

History, 26.06.2020 16:01