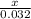Lily is preparing two samples different
compounds so that each sample contains the
same mass of calcium. The first sample she
prepares is 10.0 grams of calcium phosphate,
Ca3(PO4)2. She is preparing her second sample
using calcium hydroxide, Ca(OH)2. What mass of
Ca(OH)2 should she prepare so that it contains
the same mass of calcium as the first sample?
Help?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 14:00
8.98 dm3 of hydrogen gas is collected at 38.8 °c. find the volume the gas will occupy at -39.9 °c if the pressure remains constant.
Answers: 3

Chemistry, 22.06.2019 20:30
The activation energy for the reaction no2(g)+co2(g)⟶no(g)+co(g) is ea = 300 kj/mol and the change in enthalpy for the reaction is δh = -100 kj/mol . what is the activation energy for the reverse reaction?
Answers: 3

Chemistry, 22.06.2019 23:30
The density of benzene at 15 °c is 0.8787 g/ml. calculate the mass of 0.1500 l of benzene at this temperature. enter your answer in terms of grams
Answers: 2

Chemistry, 23.06.2019 00:00
How is the way a mixture is combined different from how a compound is combined?
Answers: 3
You know the right answer?
Lily is preparing two samples different
compounds so that each sample contains the
same...
compounds so that each sample contains the
same...
Questions

Spanish, 02.12.2020 16:20


Mathematics, 02.12.2020 16:20

Mathematics, 02.12.2020 16:20


English, 02.12.2020 16:20









Social Studies, 02.12.2020 16:20




Biology, 02.12.2020 16:20

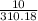
 =
=