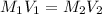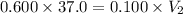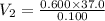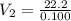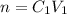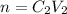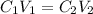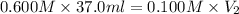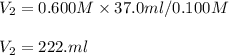Chemistry, 09.04.2020 07:30 hgghukghj1814
A 37.0 mL aliquot of a 0.600 M stock solution must be diluted to 0.100 M. Assuming the volumes are additive, how much water should be added?

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 20:30
Five students had to answer the question how are elements arranged in a periodic tabledamon: i think the elements are arranged by increasing massflo: i think the elements are arranged according to their properties sienna: i think the elements are arranged by when their discovers kyle: i think the elements are arranged according to how common they areglenda: i don't agree with any of themwho is right
Answers: 1

Chemistry, 22.06.2019 13:50
Read the chemical equation. 2c2h2 + 5o2 → 4co2 + 2h2o which of the following statements would be correct if one mole of c2h2 was used in this reaction? one mole of oxygen was used in this reaction. five moles of oxygen were used in this reaction. four moles of carbon dioxide were produced from this reaction. two moles of carbon dioxide were produced from this reaction.
Answers: 3

Chemistry, 22.06.2019 14:30
What state of matter is ice a. liquid b. element c. solid d. gas
Answers: 1

Chemistry, 22.06.2019 14:50
Complete the following statements to describe solids, liquids, and gases. select the correct answer from each drop-down menu. a solid a definite volume and a definite shape. a liquid a definite volume and a definite shape. a gas a definite volume and a definite shape
Answers: 1
You know the right answer?
A 37.0 mL aliquot of a 0.600 M stock solution must be diluted to 0.100 M. Assuming the volumes are a...
Questions




Biology, 28.01.2021 19:10

English, 28.01.2021 19:10



English, 28.01.2021 19:10


Mathematics, 28.01.2021 19:10

English, 28.01.2021 19:10

Chemistry, 28.01.2021 19:10


Mathematics, 28.01.2021 19:10



Biology, 28.01.2021 19:10