
Chemistry, 09.04.2020 19:25 sparky1234
A student puts 0.020 mol of methyl methanoate into an empty and rigid 1.0 L vessel at 450 K. The pressure is measured to be 0.74 atm. When the experiment is repeated using 0.020 mol of ethanoic acid instead of methyl methanoate, the measured pressure is lower than 0.74 atm. The lower pressure for ethanoic acid is due to the following reversible reaction. CH3COOH(g)+CH3COOH(g) ⇋ (CH3COOH)2(g)+Assume that when equilibrium has been reached, 50 percent of the ethanoic acid molecules have reacted. i. Calculate the total pressure in the vessel at equilibrium at 450 K. ii. Calculate the value of the equilibrium constant, Kp, for the reaction at 450 K

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 06:30
If 1.8 l of water is added to 2.5l of a 7.0 molarity koh solution, what is the molarity of the new solution
Answers: 1

Chemistry, 22.06.2019 08:30
What is the independent variable in this investigation? mass volume sample number substance density
Answers: 3

Chemistry, 22.06.2019 14:30
According to le chatelier’s principle, a system in chemical equilibrium responds to stress by shifting the equilibrium in a direction that reduces the stress. normalizes the stress. increases the stress. changes the stress.
Answers: 1

You know the right answer?
A student puts 0.020 mol of methyl methanoate into an empty and rigid 1.0 L vessel at 450 K. The pre...
Questions


Physics, 06.05.2021 08:20


Mathematics, 06.05.2021 08:20

Mathematics, 06.05.2021 08:20

Mathematics, 06.05.2021 08:20


Mathematics, 06.05.2021 08:20

History, 06.05.2021 08:20



Physics, 06.05.2021 08:20


Mathematics, 06.05.2021 08:20


Mathematics, 06.05.2021 08:20




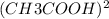 gas formed are calculated as
gas formed are calculated as



