
Chemistry, 20.09.2019 06:20 jonmorton159
Which of the following statements concerning the reaction shown below are true? p4 (s) + 3o2 (g) → p4o6 (s) ∆h = -1640 kj i. heat is absorbed ii. heat is released iii. rxn is exothermic iv. rxn is endothermic v. products have higher enthalpy content than reactants vi. reactants have higher enthalpy content than products

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 06:30
The following reaction shows the products when sulfuric acid and aluminum hydroxide react. al(oh)3 + h2so4 → al2(so4)3 + h2o the table shows the calculated amounts of reactants and products when the reaction was conducted in a laboratory. sulfuric acid aluminum hydroxide initial amount of reactant 40 g 15 g theoretical yield of water from reactant 14.69 g 10.38 g what is the approximate amount of the leftover reactant? 11.73 g of sulfuric acid 10.33 g of sulfuric acid 11.12 g of aluminum hydroxide 13.67 g of aluminum hydroxide
Answers: 3

Chemistry, 22.06.2019 10:30
Which describes fat? a: a carbohydrate that produces energy b: a nucleic acid that directs cell function c: a lipid that stores energy d: a protein that speeds up a chemical reaction
Answers: 1

Chemistry, 22.06.2019 12:30
When a scientific theory has been tested and proved by the scientific community, it becomes a law
Answers: 2

Chemistry, 22.06.2019 19:30
What is the area in square meters of 448 g ai foil that has a thickness of 23921 nm? the density is 2.70 g/cm
Answers: 3
You know the right answer?
Which of the following statements concerning the reaction shown below are true? p4 (s) + 3o2 (g) →...
Questions

Mathematics, 06.05.2021 23:00




Mathematics, 06.05.2021 23:00






Biology, 06.05.2021 23:00




History, 06.05.2021 23:00


Mathematics, 06.05.2021 23:00


Mathematics, 06.05.2021 23:00

Mathematics, 06.05.2021 23:00

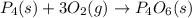
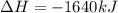
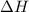 for the reaction comes out to be negative.
for the reaction comes out to be negative.

