
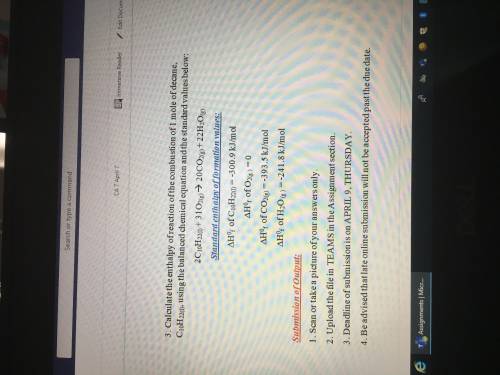
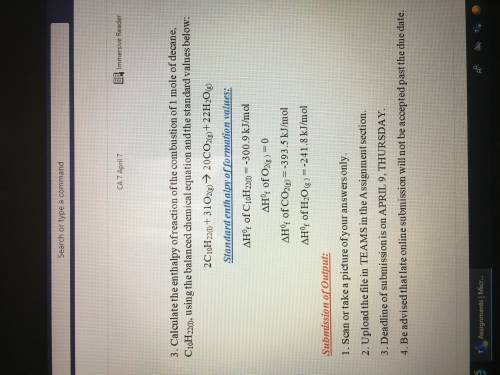
Calculate the enthalpy of the formation of butane, C4H10, using the balanced chemical equation and the standard value below:
4C(s) + 5H2(g) => C4H10(g)
Standard enthalpy of formation values:
(Delta Triangle)H^0 of C(s)= -393.5kJ/mol
(Delta triangle)H^0f of H2(g)=-285.8 kJ/mol
(Delta triangle)H^0f of C4H10(g)=-2877.6kJ/mol

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 09:30
Melissa is interested in her family tree and how her family has changed over its many generations. melissa probably more closely resembles
Answers: 2

Chemistry, 22.06.2019 13:30
1) which of the following is the best example of a physical change? a) sugar dissolving in tea b) firefly glowing 2) in the combustion of ethane, what is/are the reactants? c2h6 + o2 ==> co2 + h2o a) c2h6 and o2 b) co2 and c2h6
Answers: 2

Chemistry, 22.06.2019 15:30
Which of the following are correct values for the ideal gas laws constant r
Answers: 1

Chemistry, 22.06.2019 18:20
Categorize them by metal, nonmetal, in periodic tableductilenon-ductilemalleableoften gain electrons easilygood conductorpoor conductorcan be liquidselements
Answers: 2
You know the right answer?
Calculate the enthalpy of the formation of butane, C4H10, using the balanced chemical equation and t...
Questions


Mathematics, 17.10.2019 13:20


Social Studies, 17.10.2019 13:20

Mathematics, 17.10.2019 13:20


Mathematics, 17.10.2019 13:20

Computers and Technology, 17.10.2019 13:20


Mathematics, 17.10.2019 13:20



Health, 17.10.2019 13:20



Mathematics, 17.10.2019 13:20

Chemistry, 17.10.2019 13:20

Mathematics, 17.10.2019 13:30

Mathematics, 17.10.2019 13:30

Business, 17.10.2019 13:30




