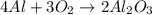Chemistry, 10.04.2020 04:56 katiedurden02
A 0.191g piece of solid aluminum reacts with gaseous oxygen from the atmosphere to form solid aluminum oxide. In the laboratory, a student weighs the mass of the aluminum oxide collected from this reaction as 0.199 g. The 0.191g solid aluminum is the .A) percent yield,
B) excess reagent,
C) limiting reagent,
D) actual yield,
E) theoretical yield

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 00:30
What must happen before a body cell can begin mitotic cell division
Answers: 2

Chemistry, 22.06.2019 09:00
Given the following reaction: c3h8+5o2=3co2+4h20 how many grams of co2 will be produced 7 g of c3h8 and 98 g of o2
Answers: 1


Chemistry, 22.06.2019 20:00
Glucose (c6h12o6) is an important biological molecule. (round the answer to nearest hundredth.) what is the percent by mass of carbon in glucose?
Answers: 2
You know the right answer?
A 0.191g piece of solid aluminum reacts with gaseous oxygen from the atmosphere to form solid alumin...
Questions

Mathematics, 05.07.2019 20:00




Biology, 05.07.2019 20:00


History, 05.07.2019 20:00

Mathematics, 05.07.2019 20:00


Arts, 05.07.2019 20:00

Social Studies, 05.07.2019 20:00




Computers and Technology, 05.07.2019 20:00


Chemistry, 05.07.2019 20:00


Mathematics, 05.07.2019 20:00