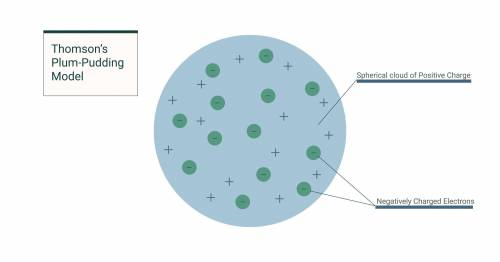
Which of the following answer choices best describes J. J. Thomson's plum-pudding model?An atom is surrounded by a firm outer shell and contains positively charged particles in its core. An atom is surrounded by a firm outer shell and contains negatively charged particles in its core. An atom consists of positively charged matter that contains negatively charged particles. An atom consists of negatively charged matter that contains positively charged particles.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 23:00
At room temperature what happens to the average kinetic energy of the molecules of a solid, liquid, and a gas
Answers: 2


Chemistry, 22.06.2019 18:30
Which of the following nuclei would be the least stable a 2 protons, 2 neutrons b 1 proton 1 neutron c 1 proton 3 neutrons d 1 proton 2 neutrons
Answers: 3

Chemistry, 22.06.2019 23:00
What is the mass of naoh that would have to be added to 500 ml of a solution of 0.20 m acetic acid in order to achieve a ph of 5.0?
Answers: 1
You know the right answer?
Which of the following answer choices best describes J. J. Thomson's plum-pudding model?An atom is s...
Questions


Mathematics, 08.11.2020 01:10




Business, 08.11.2020 01:10

English, 08.11.2020 01:10

Chemistry, 08.11.2020 01:10


English, 08.11.2020 01:10





Spanish, 08.11.2020 01:10

Mathematics, 08.11.2020 01:10

Mathematics, 08.11.2020 01:10


Mathematics, 08.11.2020 01:10

Mathematics, 08.11.2020 01:10