
Chemistry, 10.04.2020 21:57 tytybruce2
What is the activation energy of a reaction whose rate constant doubles when the temperature is increased from 300K to 350K (assuming that the activation energy and pre-exponential factor are both temperature independent)? Express your answer as a number with units.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 06:30
Select the correct text in the passage. which sentences describe examples of sustainable living? i live in an old apartment building downtown, but my company is based in an office park on the outskirts of the city. i drive an old car that needs to be replaced. i plan to buy a hybrid for better gas mileage, but for now i am able to carpool with a couple of friends from work. the drive to the office park is about 45 minutes each way, but we do get to work in a modern building. the architects just received a leed certification for the design.
Answers: 3

Chemistry, 22.06.2019 07:00
The organism shown is a free-living one that is anchored to the bottom of ponds and streams during one stage of its life cycle what is the common name for the group to which this organism belong
Answers: 3

Chemistry, 22.06.2019 10:00
How many grams of co(g) are there in 74.5 ml of the gas at 0.933 atm and 30o c?
Answers: 1

Chemistry, 22.06.2019 14:00
How is the atomic number of a nucleus changed by alpha decay
Answers: 2
You know the right answer?
What is the activation energy of a reaction whose rate constant doubles when the temperature is incr...
Questions


Mathematics, 16.04.2020 17:50



Social Studies, 16.04.2020 17:50

Mathematics, 16.04.2020 17:50







Mathematics, 16.04.2020 17:51


History, 16.04.2020 17:51

Mathematics, 16.04.2020 17:51




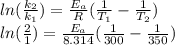
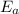 .
. kJ
kJ

