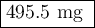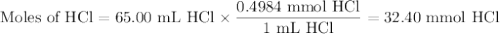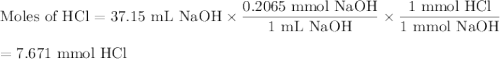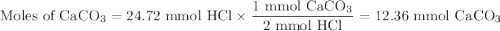Chemistry, 11.04.2020 00:53 kaylijocombs
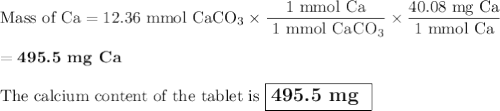
The active component in one type of calcium dietary supplement is calcium carbonate. A 1.2450 g tablet of the supplement is added to 65.00 mL of 0.4984 M HCl(aq) and allowed to react. After completion of the reaction, the excess HCl(aq) requires 37.15 mL of 0.2065 M NaOH(aq) for its titration to the equivalence point. What is the calcium content of the tablet, expressed in milligrams of Ca?

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 10:00
Why is the structure of molecule important to its function?
Answers: 1

Chemistry, 22.06.2019 18:10
Consider the following reaction at equilibrium: c(s)+h2o(g)⇌co(g)+h2(g) predict whether the reaction will shift left, shift right, or remain unchanged upon each of the following disturbances. a) c is added to the reaction mixture. b) h2ois condensed and removed from the reaction mixture c) co is added to the reaction mixture d) h2 is removed from the reaction mixture.
Answers: 3

Chemistry, 22.06.2019 20:30
The activation energy for the reaction no2(g)+co2(g)⟶no(g)+co(g) is ea = 300 kj/mol and the change in enthalpy for the reaction is δh = -100 kj/mol . what is the activation energy for the reverse reaction?
Answers: 3
You know the right answer?
The active component in one type of calcium dietary supplement is calcium carbonate. A 1.2450 g tabl...
Questions



Mathematics, 28.04.2021 17:30


English, 28.04.2021 17:30

History, 28.04.2021 17:30



Mathematics, 28.04.2021 17:30


Mathematics, 28.04.2021 17:30





Mathematics, 28.04.2021 17:30