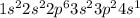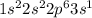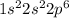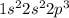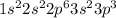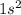Chemistry, 11.04.2020 04:03 browneyedbaby20
Which of the following electron configurations represents an excited state of the indicated atom? Group of answer choices
a. Na: 1s2 2s2 2p6 3s2 3p2 3s1
b. Ne: 1s2 2s2 2p6
c. N: 1s2 2s2 2p3
d. P: 1s2 2s2 2p6 3s2 3p2 4s1
e. He: 1s2

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 17:30
Energy defines the different "states" of matter. in no more than 3 sentences, describe the amount of kinetic energy that each of the 3 states of matter possesses and relate that to the atom/molecular motion of each "state".
Answers: 2

Chemistry, 23.06.2019 03:30
How many grams of sodium chloride are in 250ml of a 2.5m naci solution
Answers: 1

Chemistry, 23.06.2019 13:30
Where are electrons with the lowest energy found? in the nucleus farthest from the nucleus outside the atom closest to the nucleus
Answers: 1

Chemistry, 23.06.2019 15:40
The poh of a solution is 6.0. which statement is correct? use poh=-log[oh and ph+poh= 14 o o o the ph of the solution is 20.0. the concentration of oh ions is 1.0 x 10-8 m- the concentration of oh ions is 1.0 x 106 m- the ph of the solution is 8.0.
Answers: 3
You know the right answer?
Which of the following electron configurations represents an excited state of the indicated atom? Gr...
Questions



English, 05.11.2020 18:30

Chemistry, 05.11.2020 18:30










Computers and Technology, 05.11.2020 18:30





Mathematics, 05.11.2020 18:30