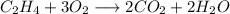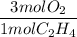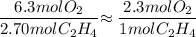C2H4 + O2 → CO2 + H2O

Chemistry, 11.04.2020 20:24 garrettadkins2002
The equation below shows the incomplete combustion of ethene.
C2H4 + O2 → CO2 + H2O
If 2.70 mol C2H4 is reacted with 6.30 mol O2 identify the limiting reagent

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 02:10
When 225mg of anthracene, c14h10(s), was burned in a bomb calorimeter the temperature rose by 1.75k. calculate the calorimeter constant. by how much will the temperature rise when 125mg of phenol, c6h5oh(s), is burned in the calorimeter under the same conditions? (δch< (c14h10,s)=–7061 kj mol−1.)
Answers: 3

Chemistry, 22.06.2019 05:30
Compare and contrast physical changes with chemical changes.
Answers: 1

Chemistry, 22.06.2019 07:00
What effect does a decrease in temperature have on the overall rate of a chemical reaction? a decrease in temperature decreases . the reaction rate will
Answers: 1

Chemistry, 22.06.2019 14:20
Which of the following are sources of revenue for media companies? a. direct sales to producers b.advertising and subscriptions c. online purchase d. capital investments
Answers: 1
You know the right answer?
The equation below shows the incomplete combustion of ethene.
C2H4 + O2 → CO2 + H2O
C2H4 + O2 → CO2 + H2O
Questions


SAT, 28.12.2021 15:10



SAT, 28.12.2021 15:10




Physics, 28.12.2021 15:10


Computers and Technology, 28.12.2021 15:10





SAT, 28.12.2021 15:20

SAT, 28.12.2021 15:20

Mathematics, 28.12.2021 15:20

SAT, 28.12.2021 15:20


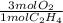
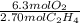 ≈
≈