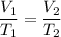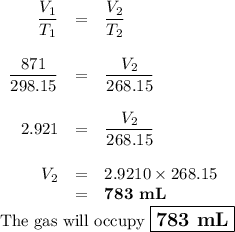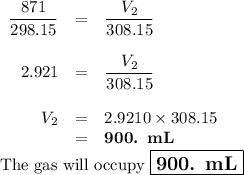Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 23:30
Write a paragraph that provides examples of each stage of volcanic activity, a description of the volcano, and facts about each stage.
Answers: 1

Chemistry, 22.06.2019 10:00
Which sentence about particles in matter is true? a. atoms are present in solids and liquids but not in gases. b. the particles of matter are in constant motion. c. the same kinds of atoms are found in different elements. d. when a solid changes to a liquid, the sizes of the particles change.
Answers: 1

Chemistry, 22.06.2019 11:30
Voltaic cells produce a positive overall charge. what does this indicate? a. the reaction is likely to be endothermic. b. the reaction is spontaneous. c. the reaction is not likely to occur. d. the reaction is not spontaneous.
Answers: 3

Chemistry, 22.06.2019 20:00
Carbon-14 undergoes radioactive decay in the reaction above. determine the type of radiation emitted in this reaction and describe what is happening to the nucleus during this reaction.
Answers: 2
You know the right answer?
A sample of O2 gas occupies a volume of 871 mL at 25 °C. If pressure remains constant, what would be...
Questions


Biology, 03.09.2020 22:01

Mathematics, 03.09.2020 22:01



Mathematics, 03.09.2020 22:01

Mathematics, 03.09.2020 22:01

Mathematics, 03.09.2020 22:01

Mathematics, 03.09.2020 22:01

Mathematics, 03.09.2020 22:01

Chemistry, 03.09.2020 22:01

History, 03.09.2020 22:01





English, 03.09.2020 22:01


Mathematics, 03.09.2020 22:01