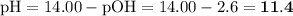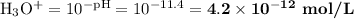Chemistry, 13.04.2020 01:10 alejandro8212003
Ammonia has a Kb of 1.8 × 10−5. Find [H3O+], [OH−], pH, and pOH for a 0.310 M ammonia solution.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 16:10
Amixture initially contains a, b, and c in the following concentrations: [a] = 0.300 m , [b] = 1.05 m , and [c] = 0.550 m . the following reaction occurs and equilibrium is established: a+2b⇌c at equilibrium, [a] = 0.140 m and [c] = 0.710 m . calculate the value of the equilibrium constant, kc.
Answers: 1

Chemistry, 22.06.2019 20:30
Consider the following unbalanced equation for the combustion of hexane: αc6h14(g)+βo2(g)→γco2(g)+δh2o(g) part a balance the equation. give your answer as an ordered set of numbers α, β, γ, use the least possible integers for the coefficients. α α , β, γ, δ = nothing request answer part b determine how many moles of o2 are required to react completely with 5.6 moles c6h14. express your answer using two significant figures. n n = nothing mol request answer provide feedback
Answers: 2


Chemistry, 23.06.2019 07:00
Write a hypothesis that answers the lesson question, “while observing a chemical reaction, how can you tell which reactant is limiting? ” hypothesis: if a substance is the limiting reactant, then . . because . .
Answers: 1
You know the right answer?
Ammonia has a Kb of 1.8 × 10−5. Find [H3O+], [OH−], pH, and pOH for a 0.310 M ammonia solution....
Questions




Mathematics, 10.10.2019 22:00

History, 10.10.2019 22:00

History, 10.10.2019 22:00

History, 10.10.2019 22:00


English, 10.10.2019 22:00

Computers and Technology, 10.10.2019 22:00


Biology, 10.10.2019 22:00


Mathematics, 10.10.2019 22:00



Mathematics, 10.10.2019 22:00


Mathematics, 10.10.2019 22:00

Mathematics, 10.10.2019 22:00

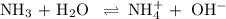
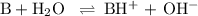
![K_{\text{b}} = \dfrac{\text{[BH}^{+}]\text{[OH}^{-}]}{\text{[B]}} = 1.8 \times 10^{-5}\\\\\dfrac{x^{2}}{0.100 - x} = 1.8 \times 10^{-5}](/tpl/images/0596/0459/4767e.png)
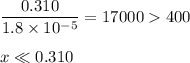
![\dfrac{x^{2}}{0.310} = 1.8 \times 10^{-5}\\\\x^{2} = 0.310 \times 1.8 \times 10^{-5}\\x^{2} = 5.58 \times 10^{-6}\\x = \sqrt{5.58 \times 10^{-6}}\\x = \text{[OH]}^{-} = \mathbf{2.4 \times 10^{-3}} \textbf{ mol/L}](/tpl/images/0596/0459/7dcd2.png)
![\text{pOH} = -\log \text{[OH}^{-}] = -\log(2.4 \times 10^{-3}) = \mathbf{2.6}](/tpl/images/0596/0459/b8dea.png)