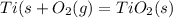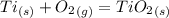Chemistry, 24.09.2019 15:50 travawnward
Write a balanced chemical equation for the reaction that occurs when titanium metal undergoes a combination reaction with o2(g).

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 01:30
Phosphorous acid, h3po3(aq) , is a diprotic oxyacid that is an important compound in industry and agriculture. the values of phosphorous acid are 1.30 6.70 calculate the ph for each of the given points in the titration of 50.0 ml of 1.5 m h3po3(aq) with 1.5 m koh(aq) .
Answers: 3

Chemistry, 22.06.2019 06:40
Three alkali metals in group 1 are a. calcium, strontium, barium b. boron, aluminum, gallium c. sodium, potassium, rubidium d. fluorine, iodine, chlorine
Answers: 1

Chemistry, 22.06.2019 14:00
How is the atomic number of a nucleus changed by alpha decay
Answers: 2

Chemistry, 23.06.2019 01:30
Which of the following statements is true about energy quantization at the atomic level? electrons in the outermost orbits are the most stable. electrons in all the orbits around the nucleus have the same amount of energy. electrons in the orbit closest to the nucleus have the least amount of energy. electrons absorb or release the same amount of energy independent of the energy levels.
Answers: 1
You know the right answer?
Write a balanced chemical equation for the reaction that occurs when titanium metal undergoes a comb...
Questions



Mathematics, 29.03.2021 18:40


Biology, 29.03.2021 18:40

Mathematics, 29.03.2021 18:40


Chemistry, 29.03.2021 18:40

Mathematics, 29.03.2021 18:40



Mathematics, 29.03.2021 18:40


Social Studies, 29.03.2021 18:40

History, 29.03.2021 18:40

English, 29.03.2021 18:40

Mathematics, 29.03.2021 18:40


Social Studies, 29.03.2021 18:40

Mathematics, 29.03.2021 18:40