Chemistry, 13.04.2020 11:27 jrfranckowiak
1) Green laser pointers are becoming popular for presentations. The green light is monochromatic
and has a wavelength of 532 nm.
a) What is the frequency of this light?
b) What is the energy in Joules of one photon of this light?
c) What is the energy in Joules of one mole of photons of this light?
d) What is the orbital energy in Joules of an electron in the ground state (n=1) in a hydrogen atom
according to the Bohr model?
e) What is the orbital energy in Joules of an electron in the n=2 state in a hydrogen atom according to
the Bohr model?


Answers: 1


Another question on Chemistry



Chemistry, 22.06.2019 18:50
Which of the following is a conclusion that resulted from ernest rutherford’s scattering experiment? (will mark brainliest) a. the nucleus is negatively charged b. the atom is a dense solid and is indivisible c. the mass is conserved when atoms react chemically d. the nucleus is very small and the atom is mostly empty space
Answers: 3

Chemistry, 22.06.2019 20:00
Carbon-14 undergoes radioactive decay in the reaction above. determine the type of radiation emitted in this reaction and describe what is happening to the nucleus during this reaction.
Answers: 2
You know the right answer?
1) Green laser pointers are becoming popular for presentations. The green light is monochromatic
Questions

Mathematics, 21.04.2021 22:20

Physics, 21.04.2021 22:20

History, 21.04.2021 22:20

Biology, 21.04.2021 22:20


History, 21.04.2021 22:20

Mathematics, 21.04.2021 22:20

Mathematics, 21.04.2021 22:20


Biology, 21.04.2021 22:20



Mathematics, 21.04.2021 22:20

Chemistry, 21.04.2021 22:20


Mathematics, 21.04.2021 22:20



Spanish, 21.04.2021 22:20

Physics, 21.04.2021 22:20

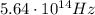

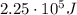
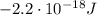
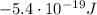
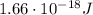
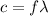
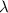 is the wavelength
is the wavelength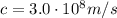 is the speed of light
is the speed of light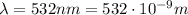 is the wavelength of the green light emitted by the laser
is the wavelength of the green light emitted by the laser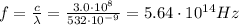
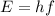
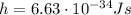 is the Planck's constant
is the Planck's constant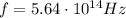 is the frequency of the photon
is the frequency of the photon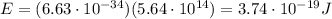
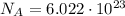

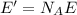
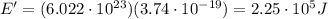
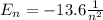 [eV]
[eV]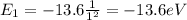
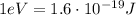
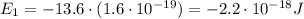
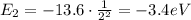
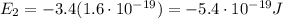
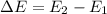
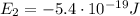 is the energy of orbital n=2
is the energy of orbital n=2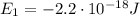 is the energy of orbital n=1
is the energy of orbital n=1