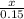Chemistry, 13.04.2020 18:33 gamerdoesart
A solution containing 20.0 g of sodium sulfite reacts with 7.0 ml of phosphoric acid. The concentration of the acid solution is such that there are 1.83 grams of H3PO4 per milliliter of solution. Determine the following: a. The mass of the excess reactant remaining at completion. b. Grams of water produced. c. Moles of sodium phosphate produced. d. Grams of sulfur dioxide produced.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 03:20
Which type of substance ionizes partially and gives off hydrogen ions when dissolved in water? a. strong acid b. strong base c. weak acid d. weak base
Answers: 1

Chemistry, 22.06.2019 15:00
Answer explain why it is not possible to deduce a complete order of reactivity.
Answers: 3

Chemistry, 22.06.2019 19:00
How many liters of ethylene glycol antifreeze (c2h6o2), with a density of 1.100 g/l, would you add to your car radiator containing 15.0 kg of water if you needed to protect your engine to - 21.5°c? for water, kf = 1.86°c m -1.
Answers: 1

Chemistry, 22.06.2019 19:30
Astring vibrates with a frequency of 10 hz. why can't a person hear the sound waves produced by the vibrating string, no matter how large the amplitude of the waves? out! this is homework and due tomorrow! you so much!
Answers: 2
You know the right answer?
A solution containing 20.0 g of sodium sulfite reacts with 7.0 ml of phosphoric acid. The concentrat...
Questions

Mathematics, 06.05.2020 07:44


Biology, 06.05.2020 07:44

Chemistry, 06.05.2020 07:44

Mathematics, 06.05.2020 07:44

Mathematics, 06.05.2020 07:44

Chemistry, 06.05.2020 07:44

Mathematics, 06.05.2020 07:44

Mathematics, 06.05.2020 07:44

Mathematics, 06.05.2020 07:44


Biology, 06.05.2020 07:44

Mathematics, 06.05.2020 07:44


Mathematics, 06.05.2020 07:44



Business, 06.05.2020 07:44


Mathematics, 06.05.2020 07:44

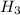 P
P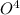 + 3
+ 3 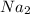
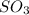 ⇒ 2
⇒ 2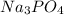 +3
+3 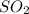 +3
+3 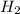 O
O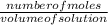
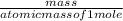
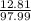
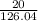
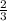 =
= 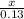
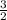 =
=