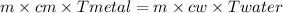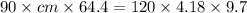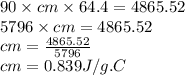Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 12:30
The missing component to the table to the right or indicated with orange letters complete the table by filling in the corresponding numbers or symbols
Answers: 3

Chemistry, 22.06.2019 14:30
Select all that apply. using a value of ksp = 1.8 x 10-2 for the reaction pbcl2 (s) pb+2(aq) + 2cl -(aq). the concentration of the products yield a ksp of 2.1 x 10-2:
Answers: 2

Chemistry, 22.06.2019 17:30
Aroller coaster is traveling at 13 mi./s when you purchase a hill that is 400 m long and down the hill exonerate at 4.0 m/s squared what is the final velocity of the posterior found your answer to the nearest number
Answers: 1
You know the right answer?
A 90.0 g piece of metal, initially at 98.6°C, is placed into 120.0 g of
water initially at 24....
water initially at 24....
Questions





Mathematics, 26.10.2020 06:30




Mathematics, 26.10.2020 06:30

Mathematics, 26.10.2020 06:30

English, 26.10.2020 06:30


Biology, 26.10.2020 06:30

Mathematics, 26.10.2020 06:30

Mathematics, 26.10.2020 06:30

English, 26.10.2020 06:30


Biology, 26.10.2020 06:30


Business, 26.10.2020 06:30

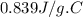 .
.