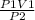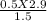Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 06:10
Explain the relationship between forward and backward reactions in equilibrium, and predict how changing the amount of a reactant (creating a tension) will affect that relationship.
Answers: 1

Chemistry, 22.06.2019 07:30
The table compares the number of electrons in two unknown neutral atoms. comparison of electrons atom number of electrons a 10 d 11 use this information to determine the number of valence electrons in the atoms. which of the following correctly compares the stability of the two atoms? both are unreactive. both are highly reactive. a is unreactive and d is reactive. a is reactive and d is unreactive.
Answers: 3


Chemistry, 22.06.2019 18:00
To apply in a gold the individual gold atoms are united to each other by means of a metallic bond. how would you use the gold block to determine the atomic radius of a gold atom?
Answers: 3
You know the right answer?
1. If a gas at 37 degrees Celcius occupies 2.9 L at a pressure of 380 torr, what will be the new
Questions

English, 20.04.2021 19:20


Mathematics, 20.04.2021 19:20


Mathematics, 20.04.2021 19:20








Mathematics, 20.04.2021 19:20

Mathematics, 20.04.2021 19:20

Mathematics, 20.04.2021 19:20

Mathematics, 20.04.2021 19:20


Mathematics, 20.04.2021 19:20

History, 20.04.2021 19:20