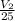Chemistry, 13.04.2020 22:29 cmflores3245
A can of coke contains 25 ML of carbon dioxide gas at 100 kpa . If you take it on a hike up Mount Everest and pressure to 50 Kpa what will the new volume of the carbon dioxide gas in your coke can be ?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 05:40
Why did southern business leaders want to increase the number of slaves
Answers: 1

Chemistry, 22.06.2019 16:50
What is conserved in the reaction shown below? h2(g) + cl2 (g) --> 2hcl(g)a. mass onlyb. mass and moles onlyc. mass, moles, and molecules onlyd. mass, moles, molecules, and volume
Answers: 2


Chemistry, 23.06.2019 05:30
Idont understand it 1.what is the boiling point of a solution of 675 grams of ethylene glycol (c2h6o2) in 2.50 liters of water?
Answers: 2
You know the right answer?
A can of coke contains 25 ML of carbon dioxide gas at 100 kpa . If you take it on a hike up Mount Ev...
Questions


Mathematics, 22.04.2021 21:20

Mathematics, 22.04.2021 21:20


Mathematics, 22.04.2021 21:20

Biology, 22.04.2021 21:20

History, 22.04.2021 21:20

Mathematics, 22.04.2021 21:20


Mathematics, 22.04.2021 21:20






Mathematics, 22.04.2021 21:20

Mathematics, 22.04.2021 21:20



Spanish, 22.04.2021 21:20

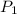 ) = 100 Kpa
) = 100 Kpa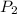 ) = 50 Kpa
) = 50 Kpa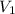 ) = 25 ml
) = 25 ml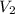 )
)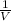
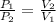
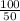 =
=