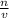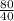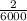80.0 grams of NaOH is dissolved in water to make a 6.00 L
solution. What is the molarity of th...


Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 06:10
Explain the relationship between forward and backward reactions in equilibrium, and predict how changing the amount of a reactant (creating a tension) will affect that relationship.
Answers: 1

Chemistry, 22.06.2019 12:30
Write the chemical formula for a compound that is made of an element from group 1 and an element from group 17
Answers: 1


Chemistry, 23.06.2019 04:00
What is the volume of 2.5 moles of nitrogen gas (n2)at standard temperature and pressure (stp)?
Answers: 1
You know the right answer?
Questions

Business, 10.02.2022 20:00

Geography, 10.02.2022 20:00




English, 10.02.2022 20:00



History, 10.02.2022 20:00


Business, 10.02.2022 20:00




Mathematics, 10.02.2022 20:00


Physics, 10.02.2022 20:00


Geography, 10.02.2022 20:00

Mathematics, 10.02.2022 20:00

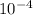 M.
M.