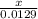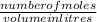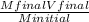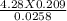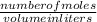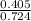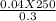Chemistry, 14.04.2020 07:44 aide1234564
1.Complete the balanced neutralization equation for the reaction below:
H2SO4 (aq) + Sr(OH)2 (aq) -->
2. How many L of a 0.209 M KI solution is needed to completely react with 2.43 g of Cu(NO3)2 according to the balanced chemical reaction:
2Cu(NO₃)₂(aq) + 4KI(aq) → 2CuI(aq) + I₂(s) + 4KNO₃(aq)
3.What volume in L of a 0.724 M Nal solution contains0.405 mol of Nal?
4. How many mL of 0.300 M NaF would be required to make a 0.0400 M solution of NaF when diluted to 250.0 mL with water?

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 21:00
Mrs. smith ordered a root beer float (vanilla ice cream + root beer). mrs. smith noticed that the three states of matter (solid, liquid, and gas) all existed simultaneously in her root beer float. a. identify each phase of matter in the root beer float. b. describe the particles of all three phases of matter in the root beer float. (how are the particles arranged and moving? ) c. identify one phase change you would see in a root beer float and described what causes this change.
Answers: 2

Chemistry, 21.06.2019 22:30
200. ml of 3.00 m nacl solution is diluted to a final volume of 500. ml. what is the molarity of the final solution?
Answers: 2


You know the right answer?
1.Complete the balanced neutralization equation for the reaction below:
H2SO4 (aq) + Sr(OH)2 (...
H2SO4 (aq) + Sr(OH)2 (...
Questions


Mathematics, 20.08.2019 05:10


Mathematics, 20.08.2019 05:10


History, 20.08.2019 05:10




Mathematics, 20.08.2019 05:20

Mathematics, 20.08.2019 05:20


Mathematics, 20.08.2019 05:20

English, 20.08.2019 05:20

Biology, 20.08.2019 05:20

History, 20.08.2019 05:20

Mathematics, 20.08.2019 05:20

Mathematics, 20.08.2019 05:20

Mathematics, 20.08.2019 05:20

Mathematics, 20.08.2019 05:20

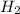 S
S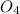 + Sr(OH)
+ Sr(OH) ⇒ SrSO4 + 2 H2O is the balanced reaction.
⇒ SrSO4 + 2 H2O is the balanced reaction.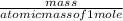
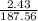
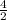 =
=