
Chemistry, 14.04.2020 16:12 buiratsamah
Butane (C4 H10(g), Hf = –125.6 kJ/mol) reacts with oxygen to produce carbon dioxide (CO2 , Hf = –393.5 kJ/mol ) and water (H2 O, Hf = –241.82 kJ/mol) according to the equation below. What is the enthalpy of combustion (per mole) of C4H10 (g)? Use .

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 20:30
There is an area in idaho named craters of the moon where most of the ground is covered with basalt, adark gray, igneous rock with no visibl crystals. what can you infer about the geographical history of the area?
Answers: 1

Chemistry, 22.06.2019 21:00
Which of the following is a physical property flammability heat of combustion solubility and toxicity
Answers: 1


Chemistry, 23.06.2019 01:00
Imagine if during the cathode ray experiment, the size of the particles of the ray was the same as the size of the atom forming the cathode. which other model or scientific observation would have also been supported? 1. this would support dalton's postulates that proposed the atoms are indivisible because no small particles are involved. 2. this would support bohr's prediction about electrons moving in orbits having specific energy. 3. this would support bohr's prediction about electrons being randomly scattered around the nucleus in the atom. 4. this would support dalton's postulates that proposed that atoms combine in fixed whole number ratios to form compounds.
Answers: 1
You know the right answer?
Butane (C4 H10(g), Hf = –125.6 kJ/mol) reacts with oxygen to produce carbon dioxide (CO2 , Hf = –393...
Questions

Mathematics, 21.03.2020 23:53




Mathematics, 21.03.2020 23:53


Health, 21.03.2020 23:53

Mathematics, 21.03.2020 23:53




Mathematics, 21.03.2020 23:53


Social Studies, 21.03.2020 23:54

Biology, 21.03.2020 23:54


Mathematics, 21.03.2020 23:55



Mathematics, 21.03.2020 23:55

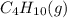 is -2657.5 kJ
is -2657.5 kJ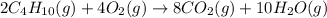
![\Delta H^o_{rxn}=[(8\times \Delta H^o_f_{CO_2(g)})+(10\times \Delta H^o_f_{H_2O(g)})]-[(1\times \Delta H^o_f_{C_4H_{10}(g)})+(4\times \Delta H^o_f_{O_2(g)})]](/tpl/images/0598/2199/66d33.png)
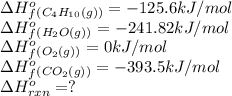
![\Delta H^o_{rxn}=[(8\times -393.5)+(10\times -241.82)]-[(2\times -125.6)+(4\times 0)]\\\\\Delta H^o_{rxn}=-5315kJ](/tpl/images/0598/2199/af1b6.png)


