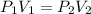Chemistry, 14.04.2020 16:33 lindasuebairdoyjpf7
A sample of an ideal gas at 1.00 atm and a volume of 1.87 L was placed in a weighted balloon and dropped into the ocean. As the sample descended, the water pressure compressed the balloon and reduced its volume. When the pressure had increased to 80.0 atm , what was the volume of the sample

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 20:30
As you move from right to left on the periodic table the atomic radius fill in the blank
Answers: 2

Chemistry, 21.06.2019 22:00
Bohr's model could only explain the spectra of which type of atoms? single atoms with one electron single atoms with more than one electron bonded atoms with one electron bonded atoms with more than one electron
Answers: 2

Chemistry, 21.06.2019 23:00
What is the molecular formula for a compound that is 46.16% carbon, 5.16% hydrogen, and 48.68% fluorine? the molar mass of the compound is 156.12 g/mol
Answers: 2

Chemistry, 22.06.2019 00:10
According to the diagram; a) identify the anode of the cell and write the half-reaction that occurs there b) write the overall equation for the reaction that occurs as the cell operates c) calculate the value of the standard cell potential ,e cell. d)write the shorthand notation of the cell above e)indicate the flow of the electrons on the diagram
Answers: 3
You know the right answer?
A sample of an ideal gas at 1.00 atm and a volume of 1.87 L was placed in a weighted balloon and dro...
Questions





Computers and Technology, 09.09.2019 21:10














Physics, 09.09.2019 21:10

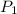 and
and 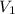 are the initial gas parameter before dropping into the ocean
are the initial gas parameter before dropping into the ocean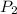 and
and 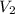 are the final gas parameter after dropping into the ocean
are the final gas parameter after dropping into the ocean