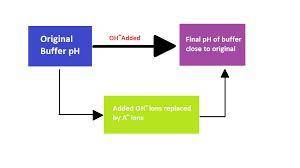
Chemistry, 14.04.2020 17:01 ashiteru123
A 1.50 L buffer solution consists of 0.118 M butanoic acid and 0.318 M sodium butanoate. Calculate the pH of the solution following the addition of 0.063 moles of NaOH . Assume that any contribution of the NaOH to the volume of the solution is negligible. The K a of butanoic acid is 1.52 × 10 − 5 .

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 13:10
Given the table below, what is the chemical formula for a compound between rb and the nitrate ion no3 -1? nitrate ion no3 -1 phosphate po4 -3 sulfate so4 -2 acetate c2h3o2 -1 ammonium nh4 +1 chromate cro4 -2 carbonate co3 -2 dichromate cr2o7 -2 permanganate mno4 -1 sulfite so3 -2 rbno3 rb2no3 rb(no3)2 rb2(no3)3
Answers: 1

Chemistry, 22.06.2019 06:00
When a spring is compressed, the energy changes from kinetic to potential. which best describes what is causing this change?
Answers: 3

Chemistry, 22.06.2019 14:00
Anthracite is so hard and pure it is also referred to as a renewable resource metamorphic rock hot bituminous coal dirty fuel
Answers: 1

You know the right answer?
A 1.50 L buffer solution consists of 0.118 M butanoic acid and 0.318 M sodium butanoate. Calculate t...
Questions

English, 31.03.2020 18:11

Computers and Technology, 31.03.2020 18:11

Mathematics, 31.03.2020 18:11

Mathematics, 31.03.2020 18:11

History, 31.03.2020 18:11





Biology, 31.03.2020 18:12


Chemistry, 31.03.2020 18:12




Mathematics, 31.03.2020 18:12

Mathematics, 31.03.2020 18:12


Mathematics, 31.03.2020 18:12