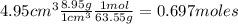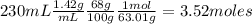Nitrogen dioxide is used industrially to produce nitric acid, but it contributes to acid rain and photochemical smog. What volume (in L) of nitrogen dioxide is formed at 735 torr and 28.2°C by reacting 4.95 cm3 of copper (d = 8.95 g/cm3 ) with 230.0 mL of nitric acid (d = 1.42 g/cm3 , 68.0% HNO3 by mass)?

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 16:00
Is this a scientific model? use complete sentences to explain why or why not. a graphic organizer showing the water cycle i need : ( asap i go it never mind
Answers: 2

Chemistry, 22.06.2019 08:30
In the millikan oil drop experiment they determined that every drop had a charge which was a while number multiple of -1.60x10^-19. if a drop has a total charge of -9.60x10^-19 then how many excess electrons are contained within the drop?
Answers: 2

Chemistry, 22.06.2019 12:00
What are the first two quantum numbers for the electrons located in subshell 4d? what are the first three quantum numbers for the electrons located in subshell 2s? how many electrons can be held in a sublevel l = 3? how many electrons can be held in the energy level n = 4? how many electrons in an atom can share the quantum numbers n = 4 and l = 3?
Answers: 1

Chemistry, 22.06.2019 21:30
Harry lives in a city, and he has a lung condition known as asthma. on certain days, harry has to stay inside because pollutants in the air make it difficult for him to breathe. which of these pollution sources are nonpoint sources that might bother harry if he goes outside? choose the two that apply.
Answers: 3
You know the right answer?
Nitrogen dioxide is used industrially to produce nitric acid, but it contributes to acid rain and ph...
Questions




Mathematics, 13.04.2021 22:10

Mathematics, 13.04.2021 22:10


Mathematics, 13.04.2021 22:10



Social Studies, 13.04.2021 22:10

Health, 13.04.2021 22:10


English, 13.04.2021 22:10

Mathematics, 13.04.2021 22:10

Mathematics, 13.04.2021 22:10

Chemistry, 13.04.2021 22:10

Mathematics, 13.04.2021 22:10

Mathematics, 13.04.2021 22:10

Spanish, 13.04.2021 22:10