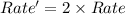Chemistry, 14.04.2020 18:03 mayamcmillan11
Consider the reaction NaCH3COO (sodium acetate in acetic acid) with tert-butyl bromide (2-bromo-2-methylpropane). If the concentration of both the nucleophile/base and the substrate are doubled, what happens to the rate of reaction?
a. it doubles
b. it quadruples
c. new rate = 1/2 of original rate
d. it does not change
e. new rate = 1/4 of original rate

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 09:40
How many grams of aluminum will there be in 98g of al2o3?
Answers: 1


Chemistry, 22.06.2019 12:30
The missing component to the table to the right or indicated with orange letters complete the table by filling in the corresponding numbers or symbols
Answers: 3

Chemistry, 22.06.2019 14:50
Consider the following multistep reaction: a b→ab(slow) a ab→a2b(fast)−−−−−−−−−−−−−−−−− 2a b→a2b(overall) based on this mechanism, determine the rate law for the overall reaction. express your answer in standard masteringchemistry format. for example, if the rate law is k[a]3[b]2 type k*[a]^3*[b]^2
Answers: 3
You know the right answer?
Consider the reaction NaCH3COO (sodium acetate in acetic acid) with tert-butyl bromide (2-bromo-2-me...
Questions



English, 19.03.2021 14:00

Mathematics, 19.03.2021 14:00


Mathematics, 19.03.2021 14:00


Mathematics, 19.03.2021 14:00


Biology, 19.03.2021 14:00


English, 19.03.2021 14:00

English, 19.03.2021 14:00


Mathematics, 19.03.2021 14:00


History, 19.03.2021 14:00


Mathematics, 19.03.2021 14:00

Chemistry, 19.03.2021 14:00

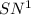 reaction and thus rate depends only on the concentration of tert-butyl bromide.
reaction and thus rate depends only on the concentration of tert-butyl bromide.![Rate=k[tertbutylbromide]^1](/tpl/images/0598/5017/a90bc.png) [base]^0
[base]^0![Rate'=k[2\times tertbutylbromide]^1[2\times base]^0}](/tpl/images/0598/5017/d2259.png)
![Rate'=k2^1[tertbutylbromide]^1[base]^0](/tpl/images/0598/5017/cfc9c.png) (2)
(2)