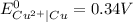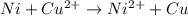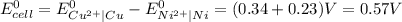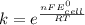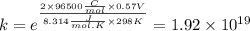Use standard reduction potentials to calculate the equilibrium constant for the reaction:
Cu²...

Chemistry, 14.04.2020 18:32 lovemichelle638
Use standard reduction potentials to calculate the equilibrium constant for the reaction:
Cu²⁺ (aq) + Ni(s) → Cu(s) + Ni²⁺ (aq)
Hint: Carry at least 5 significant figures during intermediate calculations to avoid round off error when taking the antilogarithm.

Answers: 3


Another question on Chemistry


Chemistry, 21.06.2019 21:30
Aphysical reaction is a process in which one or more reactants change into one or more products with different properties. select the best answer from the choices provided t f
Answers: 1

Chemistry, 22.06.2019 00:30
This element exists in adundance in the sun.explain how you would go about capturing sunlight.would this captured sunlight contain any of the element?
Answers: 1

Chemistry, 22.06.2019 09:00
Given the following reaction: c3h8+5o2=3co2+4h20 how many grams of co2 will be produced 7 g of c3h8 and 98 g of o2
Answers: 1
You know the right answer?
Questions

Mathematics, 26.03.2020 00:08




Computers and Technology, 26.03.2020 00:08



Mathematics, 26.03.2020 00:08

Mathematics, 26.03.2020 00:08



Mathematics, 26.03.2020 00:08









 ;
; 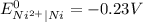
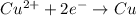 ;
;