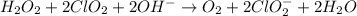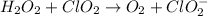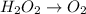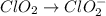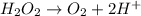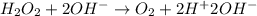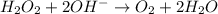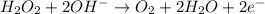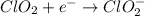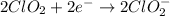Chemistry, 14.04.2020 20:14 cadereymer24
Given the partial equation: H2O2 + ClO2 → O2 + ClO2−, balance the reaction in basic solution using the half-reaction method and fill in the coefficients. The missing blanks represent H2O, H+, or OH-, as required to balance the reaction. Enter the coefficients as integers, using the lowest whole numbers. If the coefficient for something is "1", make sure to type that in and not leave it blank. Enter only the coefficients. H2O2 + ClO2 + → O2 + ClO2− +

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 01:00
Which of the following is always a reactant in a combustion reaction? oxygen nitrogen hydrogen carbon
Answers: 1

Chemistry, 22.06.2019 07:00
How heavy is thanos? a) 3000 lbs b) all of it c) the price of tea in china d) heavy enough
Answers: 2

Chemistry, 22.06.2019 12:00
An atom's configuration based on its number of electrons ends at 3p4. another atom has seven more electrons. starting at 3p, what is the remaining configuration? 3p63d34s2 3p43d54s2 3p64s23d3 3p44s23d
Answers: 3

Chemistry, 22.06.2019 14:00
Anthracite is so hard and pure it is also referred to as a renewable resource metamorphic rock hot bituminous coal dirty fuel
Answers: 1
You know the right answer?
Given the partial equation: H2O2 + ClO2 → O2 + ClO2−, balance the reaction in basic solution using t...
Questions

Mathematics, 07.12.2021 22:00


Spanish, 07.12.2021 22:00



Mathematics, 07.12.2021 22:00

Mathematics, 07.12.2021 22:00



Mathematics, 07.12.2021 22:00

Mathematics, 07.12.2021 22:00





Mathematics, 07.12.2021 22:00