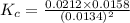Chemistry, 14.04.2020 20:01 dontworry48
Consider the following reaction: 2CH2Cl2(g) CH4(g) CCl4(g) If 0.203 moles of CH2Cl2(g), 0.323 moles of CH4, and 0.240 moles of CCl4 are at equilibrium in a 15.2 L container at 477 K, the value of the equilibrium constant, Kc, is

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 20:10
Starch and are common polysaccharide carbohydrates found in plants. sucrose glycogen fructose cellulose
Answers: 3

Chemistry, 22.06.2019 01:30
Sulfuric acid (a component of acid rain) reacts with limestone (calcium carbonate) to produce calcium sulfate and carbon dioxide. this damages buildings and statues made of limestone. which solution of sulfuric acid will damage these structures more quickly? a. 0.001% b. 0.005% c. 0.010% d. 0.015%
Answers: 3


Chemistry, 22.06.2019 19:10
How does the atmosphere to make earth livable? check all that apply. causes the seasons contains oxygen provides warmth creates important nutrients blocks harmful energy from the sun plz like !
Answers: 2
You know the right answer?
Consider the following reaction: 2CH2Cl2(g) CH4(g) CCl4(g) If 0.203 moles of CH2Cl2(g), 0.323 moles...
Questions

Mathematics, 15.04.2021 23:50

History, 15.04.2021 23:50


Mathematics, 15.04.2021 23:50

Mathematics, 15.04.2021 23:50

Geography, 15.04.2021 23:50

Mathematics, 15.04.2021 23:50

Mathematics, 15.04.2021 23:50


Mathematics, 15.04.2021 23:50







Mathematics, 15.04.2021 23:50


Mathematics, 15.04.2021 23:50

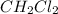 at equilibrium= 0.203 mole
at equilibrium= 0.203 mole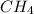 at equilibrium = 0.323 mole
at equilibrium = 0.323 mole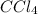 at equilibrium = 0.240mole
at equilibrium = 0.240mole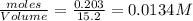
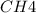 =
= 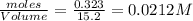
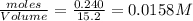
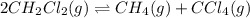
![K_c=\frac{[CH_4]\times [CCl_4]}{[CH_2Cl_2]^2}](/tpl/images/0599/0518/28fa5.png)