What is the molarity of a solution of HCl if 5.00 mL of the HCl solution is
titrated with 28.6...

Chemistry, 14.04.2020 20:43 pippyysanchezz
What is the molarity of a solution of HCl if 5.00 mL of the HCl solution is
titrated with 28.6 mL of a 0.145 M NaOH solution?
Round your answer to 3
decimal places.
HCl + NaOH → NaCl + H 2 O

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 06:00
In 1901, thomas edison invented the nickel-iron battery. the following reaction takes place in the battery. fe(s) + 2 nio(oh)(s) + 2 h2o(l) fe(oh)2(s) + 2 ni(oh)2(aq) how many mole of fe(oh)2, is produced when 5.35 mol fe and 7.65 mol nio(oh) react?
Answers: 3

Chemistry, 22.06.2019 13:30
In a ni-cd battery, a fully charged cell is composed of nickelic hydroxide. nickel is an element that has multiple oxidation states. assume the following proportions of the states: nickel charge proportions found 0 0.17 +2 0.3 +3 0.33 +4 0.5 (a) determine the mean of the nickel charge. enter the answer to 2 decimal places.(b) determine the cumulative distribution function of nickel charge.
Answers: 2


Chemistry, 23.06.2019 06:40
8. how much enthalpy/heat is transferred when 0.5113gof ammonia (nh3) reacts with excess oxygen according| to the following equation: 4nh3 +502 - 4n0+ 6h20ah = -905.4j
Answers: 1
You know the right answer?
Questions

Mathematics, 31.07.2019 20:40

Biology, 31.07.2019 20:40

History, 31.07.2019 20:40

Mathematics, 31.07.2019 20:40

Social Studies, 31.07.2019 20:40














Computers and Technology, 31.07.2019 20:40

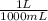 ) x (
) x (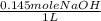 ) x (
) x (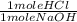 ) = 0.004147 moles HCl
) = 0.004147 moles HCl

