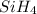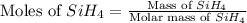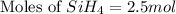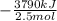Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 10:20
Gwhich r group would most likely be found in a hydrophobic area of the tertiary structure of a globular protein? which r group would most likely be found in a hydrophobic area of the tertiary structure of a globular protein? −ch2−oh −ch2−o||c−nh2 −ch2−coo− −ch2−ch2−ch2−ch2−n+h3
Answers: 3


Chemistry, 22.06.2019 13:30
An animal cell loses the ability to convert energy stored in food to energy that the cell can use. which of the cell's organelles has stopped working? a.the mitochondria b.the nucleus c.the vacuoles d.the endoplasmic reticulum
Answers: 1

Chemistry, 22.06.2019 16:50
Answer asap need by wednesday morning explain how a buffer works, using an ethanoic acid/sodium ethanoate system including how the system resists changes in ph upon addition of a small amount of base and upon addition of a small amount of acid respectively. include the following calculations in your i. calculate the ph of a solution made by mixing 25cm3 0.1m ch3cooh and 40cm3 0.1m ch3coo-na+. [ka = 1.74 x 10-5 m] ii. calculate the ph following the addition of a 10cm3 portion of 0.08 m naoh to 500cm3 of this buffer solution. iii. calculate the ph following the addition of a 10cm3 portion of 0.08 m hcl to 200cm3 of the original buffer solution.
Answers: 1
You know the right answer?
The burning of 80.3 g of SiH4 at constant pressure gives off 3790 kJ of heat. Calculate △H for this...
Questions

Chemistry, 18.03.2021 01:40

History, 18.03.2021 01:40

Mathematics, 18.03.2021 01:40

History, 18.03.2021 01:40

Mathematics, 18.03.2021 01:40

Social Studies, 18.03.2021 01:40






Mathematics, 18.03.2021 01:40

Geography, 18.03.2021 01:40

Health, 18.03.2021 01:40



History, 18.03.2021 01:40


Social Studies, 18.03.2021 01:40

Physics, 18.03.2021 01:40