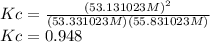Chemistry, 14.04.2020 20:55 daniellealex
At equilibrium, the concentrations of gaseous N2, O2, and NO in a sealed reaction vessel are [N2] 5 3.3 3 1023 M, [O2] 5 5.8 3 1023 M, and [NO] 5 3.1 3 1023 M. What is the value of Kc for the following reaction at the temperature of the mixture
N2(g)+O2(g) <---> 2 NO(g)

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 13:00
Imagine that you push on a large rock. at what point does your effort change the rock’s mechanical energy?
Answers: 1

Chemistry, 22.06.2019 14:10
Aconcentrated solution of ammonia is 14.8m and has a density of 0.899g/l. what is the concentration of ammonia in this solution in weight percent (%w/w)?
Answers: 1

Chemistry, 22.06.2019 14:20
Which of the following are sources of revenue for media companies? a. direct sales to producers b.advertising and subscriptions c. online purchase d. capital investments
Answers: 1

Chemistry, 22.06.2019 19:50
A2.5% (by mass) solution concentration signifies that there is a 2.5 % (by mass) solution concentration signifies that there is blank of solute in every 100 g of solution. of solute in every 100 g of solution
Answers: 3
You know the right answer?
At equilibrium, the concentrations of gaseous N2, O2, and NO in a sealed reaction vessel are [N2] 5...
Questions


Mathematics, 27.04.2021 22:00

Mathematics, 27.04.2021 22:00

Arts, 27.04.2021 22:00


Biology, 27.04.2021 22:00


English, 27.04.2021 22:00




Physics, 27.04.2021 22:00

Mathematics, 27.04.2021 22:00

Mathematics, 27.04.2021 22:00


Mathematics, 27.04.2021 22:00


Chemistry, 27.04.2021 22:00


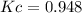
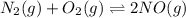
![Kc=\frac{[NO]^2}{[N_2][O_2]}](/tpl/images/0599/1972/c5383.png)