
Chemistry, 14.04.2020 21:12 larapoghosyan91
Valeric acid, HC5H9O2 (Ka = 1.5 ✕ 10−5), is used in the manufacture of magnesium valerate, a nerve-calming agent. What is the hydronium ion concentration (in M) in a 0.737 M solution of HC5H9O2? (Assume Kw = 1.01 ✕ 10−14.)

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 02:20
6. what does the symbol ah stand for? o one calorie given off by a reaction the specific heat of a substance the heat capacity of a substance the heat of reaction for a chemical reaction
Answers: 1

Chemistry, 22.06.2019 04:00
Mitosis is a type of cell division that produces cells that are identical to the parent cell. meiosis is a different type of cell division that produces cells that carry have a genetic material of the parent cell. based on the information provided how do the purpose of mitosis and meiosis differ
Answers: 3

Chemistry, 22.06.2019 08:30
Sally is making a model of a magnesium atom with an atomic mass number of 24 for her chemistry class. she has foam balls for the protons, neutrons, and electrons. she has added 6 neutrons to her model so far. how many more neutrons does she need to add to complete her neutral atom of magnesium?
Answers: 1

Chemistry, 22.06.2019 13:00
In what environment would mineral formation caused by high pressures and high temperatures most likely occur?
Answers: 3
You know the right answer?
Valeric acid, HC5H9O2 (Ka = 1.5 ✕ 10−5), is used in the manufacture of magnesium valerate, a nerve-c...
Questions

Mathematics, 19.12.2020 01:00

English, 19.12.2020 01:00

Biology, 19.12.2020 01:00

Mathematics, 19.12.2020 01:00


Law, 19.12.2020 01:00


Mathematics, 19.12.2020 01:00


Mathematics, 19.12.2020 01:00

English, 19.12.2020 01:00


English, 19.12.2020 01:00


Mathematics, 19.12.2020 01:00




Computers and Technology, 19.12.2020 01:00

Mathematics, 19.12.2020 01:00

![[H^+]=0.00332M](/tpl/images/0599/2796/eac47.png)
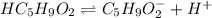
![Ka=\frac{[H^+][C_5H_9O_2^-]}{[HC_5H_9O_2]}](/tpl/images/0599/2796/6b49e.png)
 due to dissociation, it becomes:
due to dissociation, it becomes:
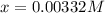
![[H^+]=x=0.00332M](/tpl/images/0599/2796/13d16.png)


