
Chemistry, 14.04.2020 21:42 jybuccaneers2022
Diiodine pentoxide (I2O5) is used in respirators to remove carbon monoxide (CO) from air according to the chemical equation: I2O5 (s) + 5 CO (g) --> I2 (s) + 5 CO2 (g) What mass of carbon monoxide could be removed from air by a respirator that contains 15.9 g of diiodine pentoxide? The molar mass of I2O5 is 333.8 g / mol.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 21:30
The density of an unknown gas at 98°c and 740 mmhg is 2.50 g/l. what is the molar mass of the gas with work showed?
Answers: 1


Chemistry, 23.06.2019 03:00
In which of the following phases of matter do molecules have the highest amount of energy? a. liquid b. gel c. solid d. gas
Answers: 2

Chemistry, 23.06.2019 03:30
Ahelium balloon contains 16.9 l of helium at stp. how many atoms of helium are in the balloon
Answers: 1
You know the right answer?
Diiodine pentoxide (I2O5) is used in respirators to remove carbon monoxide (CO) from air according t...
Questions





Mathematics, 10.05.2021 17:10


Computers and Technology, 10.05.2021 17:10

Mathematics, 10.05.2021 17:10

Mathematics, 10.05.2021 17:10

Mathematics, 10.05.2021 17:10

Social Studies, 10.05.2021 17:10

Mathematics, 10.05.2021 17:10

Mathematics, 10.05.2021 17:10


Arts, 10.05.2021 17:10


Mathematics, 10.05.2021 17:10


Arts, 10.05.2021 17:10

English, 10.05.2021 17:10

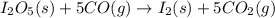
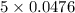 of CO from air
of CO from air

