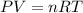Chemistry, 29.09.2019 08:30 RickandMorty420710
If the pressure, volume, and the number of moles of a gas are known, which is needed to calculate the universal gas constant from the ideal gas law?
the temperature of the gas
the molar volume of the gas
the molar mass of the gas
the partial pressure of the gas

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 18:30
In a sample of oxygen gas at room temperature, the average kinetic energy of all the balls stays constant. which postulate of kinetic molecular theory best explains how this is possible?
Answers: 2

Chemistry, 22.06.2019 09:00
If you chip a tooth, most likely you will go to the dentist to have the missing material filled in. currently the material used to fill in teeth is a polymer that is flexible when put in, yet is hardened to the strength of a tooth after irradiation with blue light at a wavelength of 461 nm. what is the energy in joules for a photon of light at this wavelength?
Answers: 1

Chemistry, 22.06.2019 12:00
Which statement best explains the relationship between an area is geography and the temperature of its surface water
Answers: 1

Chemistry, 22.06.2019 15:10
Which statement describes the phase change that occurs when dry ice is placed in an open container at room temperature?
Answers: 1
You know the right answer?
If the pressure, volume, and the number of moles of a gas are known, which is needed to calculate th...
Questions

Mathematics, 03.02.2020 09:46

History, 03.02.2020 09:46

History, 03.02.2020 09:46

Mathematics, 03.02.2020 09:46

English, 03.02.2020 09:46








Arts, 03.02.2020 09:47

Mathematics, 03.02.2020 09:47

English, 03.02.2020 09:47


Mathematics, 03.02.2020 09:47

History, 03.02.2020 09:47


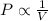 (At constant temperature and number of moles)
(At constant temperature and number of moles)
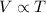 (At constant pressure and number of moles)
(At constant pressure and number of moles)
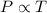 (At constant volume and number of moles)
(At constant volume and number of moles)
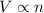 (At constant temperature and pressure)
(At constant temperature and pressure)