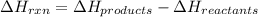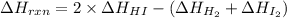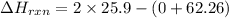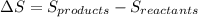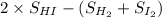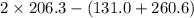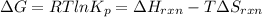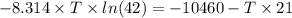Chemistry, 14.04.2020 21:54 fjjjjczar8890
Find the temperature at which Kp = 42.0 for the reaction
H2(g) + I2(g) → 2HI(g)
[Given: at 25°C, for H2(g), ∆H°f = 0, S° = 131.0 J/mol·K; for I2(g), ∆H°f = 62.26 kJ/mol, S° = 260.6 J/mol·K; for HI(g), ∆H°f = 25.9 kJ/mol, S° = 206.3 J/mol·K; assume that ∆H° and ∆S° are independent of temperature.]

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 03:30
If a solution is considered basic, then a) the hydroxide ion and hydronium ion concentrations are equal. b) the hydroxide ion concentration is less than the hydronium ion concentration. c) the hydronium ion concentration is greater than the hydroxide ion concentration. d) the hydroxide ion concentration is greater than the hydronium ion concentration.
Answers: 1

Chemistry, 22.06.2019 12:00
A5.000 g sample of niso4 h2o decomposed to give 2.755 g of anhydrous niso4. what is the formula of the hydrate? what is the full chemical name for the hydrate? what is the molar mass of the hydrate? niso4•_h2o what is the mass % of water in the hydrate?
Answers: 1

Chemistry, 23.06.2019 00:00
Exit what is the density of an object having a mass of 5.0 g and a volume of 45.0 cm3?
Answers: 1

You know the right answer?
Find the temperature at which Kp = 42.0 for the reaction
H2(g) + I2(g) → 2HI(g)
<...
H2(g) + I2(g) → 2HI(g)
<...
Questions





History, 27.04.2021 01:00

Mathematics, 27.04.2021 01:00


English, 27.04.2021 01:00

Mathematics, 27.04.2021 01:00


Mathematics, 27.04.2021 01:00

Mathematics, 27.04.2021 01:00



Mathematics, 27.04.2021 01:00

Mathematics, 27.04.2021 01:00

Mathematics, 27.04.2021 01:00


History, 27.04.2021 01:00