

Answers: 1


Another question on Chemistry

Chemistry, 23.06.2019 01:00
If a sample of radioactive isotopes takes 600 minutes to decay from 400 grams to 50 grams, what is the half-life of the isotope?
Answers: 1

Chemistry, 23.06.2019 10:30
Most ionic compouds are crystalline solids at room temperature. true falseionic compounds are electrically neutral. true falseionic compounds generally have low melting points. true falsewhen melted, ionic compounds do not conduct electricity. true falsethe electrostatic attraction between an anion and a cation is an ionic bond. true false
Answers: 1

Chemistry, 23.06.2019 14:00
What can happen to an atoms electrons when an electric current is passed through the atom?
Answers: 1

Chemistry, 23.06.2019 16:00
Fecl3 + naoh → nacl + fe(oh)3 what are the coefficients that should be added to balance this equation? use complete sentences to explain your answer. explain how this chemical reaction demonstrates the conservation of mass.
Answers: 1
You know the right answer?
Calculate the concentration of H3O in a solution that contains 5.5 × 10-5 M OH at 25°C. Identify the...
Questions

Mathematics, 01.06.2021 17:00

Chemistry, 01.06.2021 17:00


Biology, 01.06.2021 17:00


Biology, 01.06.2021 17:00





Mathematics, 01.06.2021 17:00

Chemistry, 01.06.2021 17:00

Geography, 01.06.2021 17:00

English, 01.06.2021 17:00

Mathematics, 01.06.2021 17:00

Mathematics, 01.06.2021 17:10




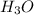 is 1.8×10⁻¹⁰ M and it is basic in nature.
is 1.8×10⁻¹⁰ M and it is basic in nature.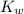 =
= 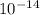 (at 25°C)
(at 25°C)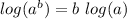 ]
]![\Rightarrow [H_3O^+]=\frac{10^{-14}}{5.5\times 10^{-5}}](/tpl/images/0599/5597/3fdf6.png)


