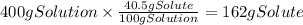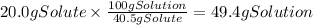Chemistry, 14.04.2020 22:30 hannahblank2466
A chemistry student needs of acetic acid for an experiment. He has available of a w/w solution of acetic acid in acetone. Calculate the mass of solution the student should use. If there's not enough solution, press the "No solution" button. Round your answer to significant digits.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 20:50
Which real-world scenarios below represent physical and chemical changes? -running a car -exploding fireworks -mixing water and powdered drink mix -combining oil and vinegar to make salad dressing -taking aspirin for a headache -diluting bleach with water-digesting dinner-spreading peanut butter on bread
Answers: 2

Chemistry, 22.06.2019 04:30
How much energy is made when a pice of wood burns. how do you know
Answers: 2

Chemistry, 22.06.2019 10:00
The reactions shown here can be combined to make the overall reaction c(s) + h2o(g) ⇌ co(g) + h2(g) by reversing some and/or dividing all the coefficients by a number. a. c(s) + o2(g) → co2(g) k=1.363×10^69 b. 2 h2(g) + o2(g) → 2 h2o(g) k=1.389×10^80 c. 2co(g) + o2 (g) → 2 co2(g) k=1.477×10^90
Answers: 1

Chemistry, 22.06.2019 11:40
Which type of precipitation would most likely form when the surface air temperature is slightly below freezing and the air temperature increases as you move upward away from the ground?
Answers: 2
You know the right answer?
A chemistry student needs of acetic acid for an experiment. He has available of a w/w solution of ac...
Questions


Biology, 05.05.2020 14:17


Mathematics, 05.05.2020 14:17

Mathematics, 05.05.2020 14:17

Social Studies, 05.05.2020 14:17

Mathematics, 05.05.2020 14:17



Mathematics, 05.05.2020 14:17


Mathematics, 05.05.2020 14:17




Mathematics, 05.05.2020 14:17



Mathematics, 05.05.2020 14:17

Mathematics, 05.05.2020 14:17