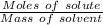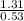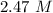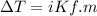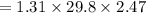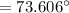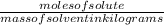Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 03:30
What is the number of moles of chemical units represented by 9.03x10^24? and how do i show work? (dumb it down )
Answers: 1

Chemistry, 22.06.2019 04:40
*will mark you brainliest + 15 points ** why does the equilibrium of a system shift when the pressure is increased? a. to maximize the stress on the system b. to stop restoring equilibrium to the system c. to increase the total moles of gas in the system d. to decrease the total moles of gas in the system
Answers: 3

Chemistry, 22.06.2019 10:00
Why is the structure of molecule important to its function?
Answers: 1
You know the right answer?
What is the freezing point of a solution made with 1.31 mol of CHCl3 in 530.0 g of CCl4 (Kf =29.8 de...
Questions

English, 22.09.2020 16:01

Mathematics, 22.09.2020 16:01


Mathematics, 22.09.2020 16:01

English, 22.09.2020 16:01


Physics, 22.09.2020 16:01


Mathematics, 22.09.2020 16:01



Mathematics, 22.09.2020 16:01

Business, 22.09.2020 16:01

Mathematics, 22.09.2020 16:01


Mathematics, 22.09.2020 16:01


Mathematics, 22.09.2020 16:01


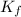 = 29.8° C/m
= 29.8° C/m