

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 23:50
2points why do scientists need governmental funding? o a. government politicians ask all the important scientific questions. o b. scientists have to pay taxes to the government on the money they make. o c. the cost of doing scientific research can be very high. o d. the government is controlled by scientists. submit
Answers: 3

Chemistry, 22.06.2019 18:00
Which three statements represent the benefits of performing experiments using computer simulations?
Answers: 3

Chemistry, 22.06.2019 22:30
Which statement best summarizes the importance of ernest rutherford’s gold foil experiment? it proved that all of john dalton’s postulates were true. it verified j. j. thomson’s work on the atomic structure. it showed that an electron circles a nucleus in a fixed-energy orbit. it showed that a nucleus occupies a small part of the whole atom.
Answers: 1

Chemistry, 22.06.2019 23:00
What is the most common reason for matter changing its state?
Answers: 1
You know the right answer?
Lithium and nitrogen react in a combination reaction to produce lithium nitride: 6Li(s) + N2(g) → 2L...
Questions

Chemistry, 12.04.2021 21:40

Mathematics, 12.04.2021 21:40










History, 12.04.2021 21:40








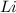 are needed to produce 0.45 mol of
are needed to produce 0.45 mol of 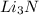
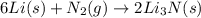
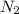 is the excess reagent.
is the excess reagent.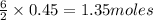 of
of 

