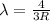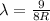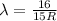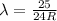Chemistry, 14.04.2020 23:42 leannaadrian
The Balmer series consists of the spectral lines from hydrogen for an electron making a transition from an excited state to the m = 2 state. The Lyman series consists of the spectral lines from hydrogen for an electron making a transition from an excited state to the m = 1 state. Determine the wavelengths of the first four spectral lines of the Lyman series (n = 2, 3, 4, and 5)

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 06:00
This flow chart shows the amount of energy that is emitted by each type of light. ultraviolet > blue light > yellow light > red light (maximum energy) (minimum energy) in an experiment, shining which type of light on a strip of metal would be least likely to produce the photoelectric effect? ultraviolet light dim blue light bright red light bright yellow light
Answers: 2

Chemistry, 22.06.2019 20:30
Select all the correct answers.which compounds have the empirical formula ch20? (multiple answers)a.c2h4o2b.c3h603c.ch2o2d.c5h1005e.c6h1206
Answers: 2

Chemistry, 22.06.2019 22:30
Gusing the milligrams of ascorbic acid you entered above, the ratio of total sample volume to aliquot volume, and the total milligrams of the vitamin c tablet that you dissolved, calculate the mass of ascorbic acid in the vitamin c tablet for each trial. do this by scaling up to find the amount (mg) of ascorbic acid in your 250 ml flask. enter your calculated mass of ascorbic acid in the vitamin c tablet, for each trial. be sure to enter your calculated mass in the corresponding order that you entered your milligrams of ascorbic acid. the milligrams of ascorbic acid you entered for entry #1 previously should correspond to the mass of ascorbic acid that you enter for entry #1 here.
Answers: 1

Chemistry, 23.06.2019 01:00
Aman applies a force of 500n to push a truck 100m down the street how much does he do?
Answers: 1
You know the right answer?
The Balmer series consists of the spectral lines from hydrogen for an electron making a transition f...
Questions



English, 22.07.2019 01:30

Computers and Technology, 22.07.2019 01:30




Mathematics, 22.07.2019 01:30

Advanced Placement (AP), 22.07.2019 01:30

Mathematics, 22.07.2019 01:30


English, 22.07.2019 01:30


Mathematics, 22.07.2019 01:30


Social Studies, 22.07.2019 01:30

History, 22.07.2019 01:30



Chemistry, 22.07.2019 01:30

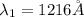
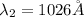
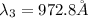
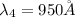
![\frac{1}{\lambda} =RZ^2[\frac{1}{n_1^2} -\frac{1}{n_2^2} ]](/tpl/images/0600/0318/c471c.png)
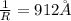 =rydberg constant
=rydberg constant