
Chemistry, 14.04.2020 23:47 sgslayerkingminecraf
The rate of a hypothetical reaction involving L and M is found to double when the concentration of L is doubled and to increase fourfold when the concentration of M is doubled. Write the rate law for this reaction.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 06:00
When a spring is compressed, the energy changes from kinetic to potential. which best describes what is causing this change?
Answers: 3

Chemistry, 22.06.2019 08:00
Asap! will give brainiest when a heat wave strikes a region causing more people to run air-conditioning units, electrical demand increases. what needs to be done to meet this increased demand? raising the control rodslowering the control rodsremoving the control rods
Answers: 1


Chemistry, 22.06.2019 19:50
What is the wavelength of a wave with a velocity of 50 m/s and a frequency of 5hz a 250 m b 0.1 m c 10m d 0.01 m
Answers: 2
You know the right answer?
The rate of a hypothetical reaction involving L and M is found to double when the concentration of L...
Questions

Biology, 06.12.2020 18:10

Social Studies, 06.12.2020 18:10


German, 06.12.2020 18:10



Mathematics, 06.12.2020 18:10

Chemistry, 06.12.2020 18:10

Mathematics, 06.12.2020 18:10


Mathematics, 06.12.2020 18:10


History, 06.12.2020 18:10


English, 06.12.2020 18:10



Mathematics, 06.12.2020 18:20


Computers and Technology, 06.12.2020 18:20

![Rate=k[L]^1[M]^2](/tpl/images/0600/0548/edc82.png)
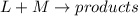
![Rate=k[L]^x[M]^y](/tpl/images/0600/0548/8fe10.png) (1)
(1)![2\times Rate=k[2L]^x[M]^y](/tpl/images/0600/0548/f32b0.png) (2)
(2)![4\times Rate=k[L]^x[2M]^y](/tpl/images/0600/0548/866b5.png) (3)
(3)![\frac{2\times Rate}{Rate}=\frac{k[2L]^x[M]^y}{k[L]^x[M]^y}](/tpl/images/0600/0548/76345.png)
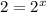
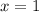
![\frac{4\times Rate}{Rate}=\frac{k[L]^x[2M]^y}{k[L]^x[M]^y}](/tpl/images/0600/0548/beaf5.png)
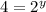
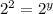
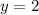
![k[L]^1[M]^2](/tpl/images/0600/0548/2ee9e.png)


